Arcturus Therapeutics Reports New Data and Provides Additional Updates from ARCT-154 and ARCT-165 Clinical Trials
Preliminary data from ongoing clinical booster study of ARCT-154 (5 mcg) shows a 50-fold increase in neutralizing antibody geometric mean concentration against SARS-CoV-2 using a validated pseudovirus microneutralization (MNT) assay
Additional data shows activity against several variants of concern and variants of interest upon boosting with ARCT-154 (5 mcg) and ARCT-165 (5 mcg) in a surrogate virus neutralization (sVNT) assay
Company to evaluate sera from ARCT-154 and ARCT-165 vaccinated participants for activity against the omicron variant; initial data anticipated Q1 2022
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211216005490/en/
ARCT-154 and ARCT-165 are being studied in a Phase 1/2 trial, sponsored by Arcturus, in
Preliminary immunogenicity results from the first eight of 12 participants administered ARCT-154 and the first nine of 12 administered ARCT-165 as a booster following initial vaccination with Comirnaty® in the ongoing Phase 1/2 study demonstrates an encouraging increase in neutralizing antibody titers following booster vaccination, as measured by a validated pseudovirus (D614G variant) microneutralization (MNT) assay as well as in an exploratory surrogate virus neutralization assay (sVNT) assessing responses to multiple variants of concern and variants of interest, including the alpha, beta, gamma and delta strains.
“The booster data showing a rise in neutralizing antibody levels and potentially broad coverage across variants, while preliminary, is encouraging and provides support for the continued development of our next-generation self-amplifying mRNA vaccine candidates as differentiated, low-dose vaccines that may be effective boosters for continued prevention of infections caused by variants of concern,” said
In the ARCT-154 and ARCT-165 arms of the booster cohort of the ongoing Phase 1/2 study being conducted in the
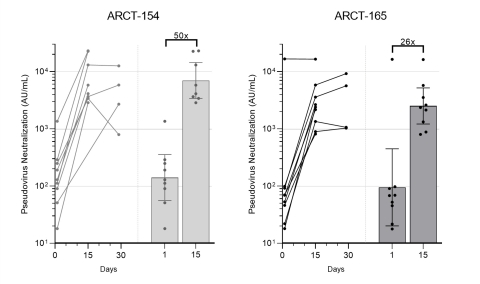
Figure 1: Pseudovirus (D614G variant) microneutralization (MNT) assay results. Virus neutralization concentrations (arbitrary units per milliliter, AU/mL) for participants at Day 1 (prior to boosting), and Days 15 and 29 after boosting with ARCT-154 (left; n = 8/12) and ARCT-165 (right; n = 9/12). Within each panel, the left graphic shows values from individuals, and the right graphic shows the geometric means of neutralization concentrations, with 95% confidence intervals. The multiples are geometric mean-fold rises (GMFR) of neutralization concentrations on Day 15 over Day 1 values. Geometric mean for Day 29 is not shown here as data from only four participants are available for this time point.
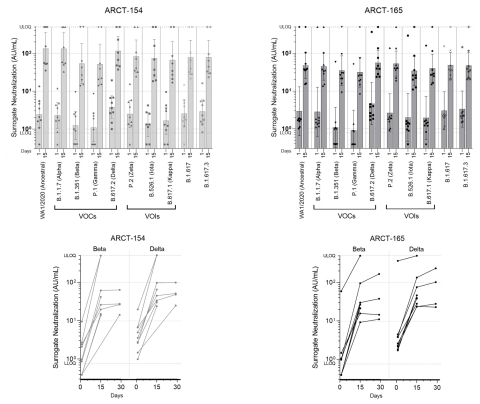
Figure 2: Surrogate virus neutralization (sVNT) assay results for SARS-CoV-2 variants. Top panels show geometric mean concentrations and 95% confidence intervals on Day 1 (prior to boosting) and Day 15 post-boost administration with ARCT-154 (left; n = 8/12) and ARCT-165 (right; n = 9/12). The bottom panels show the results for individual participants for the beta and delta variants on Day 1 (prior to boosting), and Days 15 and 29 post-boost. Geometric mean for Day 29 is not shown as data from only four participants are available for this time point. VOC: variant of concern; VOI: variant of interest; ULOQ: upper limit of quantification; LLOQ: lower limit of quantification.
Arcturus and Vinbiotech anticipate commencing the submission of regulatory documents to the
Arcturus also plans to evaluate sera from ARCT-154 and ARCT-165 vaccinated participants for activity against the SARS-CoV-2 omicron variant and expects to obtain preliminary data in the first quarter of 2022.
About
Founded in 2013 and based in
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the expectations for or likelihood of success of any collaborations, the likelihood of success (including safety and efficacy) of the Company’s pipeline (including ARCT-154 and ARCT-165), the Company’s efforts to develop a vaccine against COVID-19 and therapeutic potential thereof based on the Company’s mRNA therapeutics, the planned initiation, design or completion of experimental or preclinical work (including the evaluation of vaccine candidates against the omicron variant and other COVID-19 variants), the planned initiation, design or completion of clinical trials (including the timely completion of the Phase 1/2/3 study of ARCT-154), the likelihood that the Company will obtain clearance from regulatory authorities to proceed with future planned clinical trials, the likelihood that subsequent data from a study will be consistent with preliminary data (including with respect to the preliminary immunogenicity data of ARCT-154 and ARCT-165), the likelihood that preclinical or clinical data will be predictive of future clinical results or efficacy or safety of a candidate (including with respect to the immunogenicity results of ARCT-154 and ARCT-165 as a booster in the ongoing Phase 1/2 study), the ability to enroll, and timing for enrollment of, subjects in clinical trials, the timing and nature of any study results (including the results of the Phase 1/2/3 study in
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211216005490/en/
IR and Media Contacts
(858) 900-2682
IR@ArcturusRx.com
Kendall Investor Relations
(617) 914-0008
ctanzi@kendallir.com
Source: