Arcturus Therapeutics Announces Third Quarter 2022 Financial Update and Pipeline Progress
Entered into global partnership with CSL to develop and commercialize self-amplifying mRNA vaccines targeting COVID-19, influenza, additional pathogens, and pandemic preparedness with
BARDA award announced for up to
New LUNAR-CF preclinical data demonstrates effective delivery of ARCT-032 with LUNAR®, resulting in functional restoration of chloride ion current in CF subject bronchial epithelial cells
Investor conference call at
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221109006003/en/
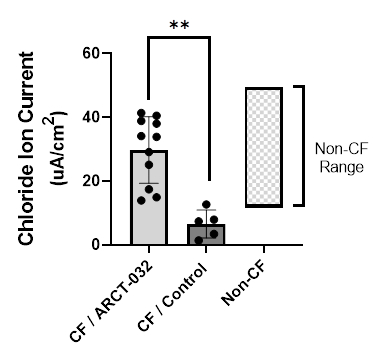
Figure: Bronchial epithelial cells (BECs) obtained from human CF donors were treated with ARCT-032 and demonstrated robust restoration of CFTR activity (chloride ion current) vs. control BECs obtained from human non-CF donors. (Graphic: Business Wire)
"Arcturus continues to execute on the promise of our next-generation self-amplifying mRNA vaccine franchise through engagements with CSL and BARDA," said
Recent Corporate Highlights
-
Announced a strategic collaboration with CSL Seqirus, one of the top two global influenza vaccine companies, for the development, manufacture, and commercialization of mRNA-based vaccines. Arcturus will receive
$200 million upfront, up to$4.3 billion in potential development and commercial milestones, 40% profit sharing for COVID-19 vaccines and up to double digit royalties for influenza and three additional respiratory infectious disease vaccines. The collaboration combines CSL Seqirus’ established global vaccine commercial and manufacturing infrastructure with Arcturus’ expertise in mRNA design and modification, LUNAR® lipid nanoparticle (LNP) technology and manufacturing know-how. Previously reported clinical results from ongoing ARCT-154 studies have demonstrated a favorable efficacy and safety profile with sustained neutralizing antibodies against COVID-19, including recent variants of concern. -
The
Biomedical Advanced Research and Development Authority (BARDA) provided Arcturus with an award valued at up to$63.2 million over three years to support preclinical, manufacturing, nonclinical safety studies, along with development and regulatory support for Arcturus’ self-amplifying mRNA vaccine platform technology for rapid pandemic influenza response through Phase 1 clinical studies. - ARCT-810, the Company’s mRNA therapeutic candidate for OTC deficiency is being evaluated in a randomized, double-blind, placebo-controlled, nested single and multiple ascending dose Phase 2 study in 24 adolescents and adults with OTC deficiency. Participating sites have identified several dozen patients in pre-screening with dosing to begin Q4 2022. All subjects in the Phase 1b single ascending dose (SAD) study have completed dosing, including the cohort dosed at 0.4 mg/kg, without requiring steroid co-treatment. The Company will share interim ARCT-810 clinical data when we announce additional liver therapeutic programs in 2023.
- New preclinical data from ARCT-032, the Company’s inhaled mRNA therapeutic for cystic fibrosis, demonstrated effective delivery of mRNA to bronchial and tracheal epithelial cells in the presence of CF sputum utilizing Arcturus’ proprietary LUNAR® technology (CF Ferret G551D model). Additional in vitro data demonstrated robust restoration of CFTR activity. Bronchial epithelial cells (BECs) obtained from human CF donors were treated with ARCT-032 and exhibited a significant increase in chloride ion current compared to control BECs obtained from non-CF donors (see Figure). ARCT-032 remains on track for CTA filing by year end 2022.
Financial Results for Third Quarter Ended
Revenues in conjunction with strategic alliances and collaborations: Arcturus’ primary sources of revenues were from consulting and related technology transfer fees, reservation fees, license fees and collaborative payments received from research and development arrangements with pharmaceutical and biotechnology partners. For the three months ended
Operating expenses: Total operating expenses for the three months ended
Research and development expenses: Research and development expenses for the three months ended
Net Loss: For the three months ended
Cash Position: The Company’s cash balance totaled
Earnings Call:
Domestic: 1-888-204-4368
International: 1-323-994-2093
Conference ID: 2581187
Webcast: Link
About
Founded in 2013 and based in
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the likelihood of success of the Company’s pipeline (including ARCT-032, ARCT-810 and ARCT-154) the expectations for beginning the collaboration with CSL Seqirus, including receiving clearance under the Hart-Scott-Rodino Antitrust Improvements Act and satisfying other closing conditions, or the likelihood of success of the collaboration with CSL Seqirus or any collaborations including the achievement of any milestones or other payments, the future activities under and fulfillment of the Company’s contract with BARDA, the ability of the Company’s influenza vaccine program to support
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks and trade names in this announcement are the property of their respective owners.
|
CONDENSED CONSOLIDATED BALANCE SHEETS |
||||||||
|
|
|
|
|
|
|
|
||
|
(in thousands, except par value information) |
|
(unaudited) |
|
|
|
|
||
|
Assets |
|
|
|
|
|
|
||
|
Current assets: |
|
|
|
|
|
|
||
|
Cash and cash equivalents |
|
$ |
237,676 |
|
|
$ |
370,492 |
|
|
Accounts receivable |
|
|
2,044 |
|
|
|
3,367 |
|
|
Prepaid expenses and other current assets |
|
|
6,960 |
|
|
|
5,102 |
|
|
Total current assets |
|
|
246,680 |
|
|
|
378,961 |
|
|
Property and equipment, net |
|
|
11,347 |
|
|
|
5,643 |
|
|
Operating lease right-of-use asset, net |
|
|
33,519 |
|
|
|
5,618 |
|
|
Equity-method investment |
|
|
— |
|
|
|
515 |
|
|
Non-current restricted cash |
|
|
2,081 |
|
|
|
2,077 |
|
|
Total assets |
|
$ |
293,627 |
|
|
$ |
392,814 |
|
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
|
Current liabilities: |
|
|
|
|
|
|
||
|
Accounts payable |
|
$ |
17,962 |
|
|
$ |
10,058 |
|
|
Accrued liabilities |
|
|
25,529 |
|
|
|
23,523 |
|
|
Current portion of long-term debt |
|
|
27,702 |
|
|
|
22,474 |
|
|
Deferred revenue |
|
|
4,656 |
|
|
|
43,482 |
|
|
Total current liabilities |
|
|
75,849 |
|
|
|
99,537 |
|
|
Deferred revenue, net of current portion |
|
|
5,179 |
|
|
|
19,931 |
|
|
Long-term debt, net of current portion |
|
|
32,038 |
|
|
|
40,633 |
|
|
Operating lease liability, net of current portion |
|
|
31,218 |
|
|
|
4,502 |
|
|
Other non-current liabilities |
|
|
3,676 |
|
|
|
— |
|
|
Total liabilities |
|
$ |
147,960 |
|
|
$ |
164,603 |
|
|
Stockholders’ equity |
|
|
|
|
|
|
||
|
Common stock, |
|
|
26 |
|
|
|
26 |
|
|
Additional paid-in capital |
|
|
601,129 |
|
|
|
575,675 |
|
|
Accumulated deficit |
|
|
(455,488 |
) |
|
|
(347,490 |
) |
|
Total stockholders’ equity |
|
|
145,667 |
|
|
|
228,211 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
293,627 |
|
|
$ |
392,814 |
|
|
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS (unaudited) |
||||||||||||
|
|
|
Three Months Ended |
|
|||||||||
|
|
|
|
|
|
|
|
||||||
|
(in thousands, except per share data) |
|
2022 |
|
|
2021 |
|
|
2022 |
|
|||
|
Revenue |
|
$ |
13,369 |
|
|
$ |
2,437 |
|
|
$ |
27,093 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|||
|
Research and development, net |
|
|
37,688 |
|
|
|
45,398 |
|
|
|
38,189 |
|
|
General and administrative |
|
|
12,488 |
|
|
|
10,860 |
|
|
|
10,993 |
|
|
Total operating expenses |
|
|
50,176 |
|
|
|
56,258 |
|
|
|
49,182 |
|
|
Loss from operations |
|
|
(36,807 |
) |
|
|
(53,821 |
) |
|
|
(22,089 |
) |
|
Loss from equity-method investment |
|
|
— |
|
|
|
(250 |
) |
|
|
(131 |
) |
|
Gain from foreign currency |
|
|
1,862 |
|
|
|
506 |
|
|
|
1,217 |
|
|
Finance expense, net |
|
|
(321 |
) |
|
|
(519 |
) |
|
|
(560 |
) |
|
Net loss |
|
$ |
(35,266 |
) |
|
$ |
(54,084 |
) |
|
$ |
(21,563 |
) |
|
Net loss per share, basic and diluted |
|
$ |
(1.33 |
) |
|
$ |
(2.05 |
) |
|
$ |
(0.82 |
) |
|
Weighted-average shares outstanding, basic and diluted |
|
|
26,467 |
|
|
|
26,338 |
|
|
|
26,425 |
|
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|||
|
Net loss |
|
$ |
(35,266 |
) |
|
$ |
(54,084 |
) |
|
$ |
(21,563 |
) |
|
Comprehensive loss |
|
$ |
(35,266 |
) |
|
$ |
(54,084 |
) |
|
$ |
(21,563 |
) |
View source version on businesswire.com: https://www.businesswire.com/news/home/20221109006003/en/
IR and Media Contacts
IR@arcturusrx.com
Kendall Investor Relations
(617) 914-0008
ctanzi@kendallir.com
Source: