Arcturus Therapeutics Announces Second Quarter 2023 Financial Update and Pipeline Progress
ARCT-154 Phase 3 COVID-19 booster trial achieved primary endpoint demonstrating strong immune response and favorable safety profile
New ARCT-154 booster clinical data demonstrate one-year durability across a panel of variants
Received FDA Fast Track Designation and Rare Pediatric Disease Designation for ARCT-810 for OTC deficiency
Received regulatory approval of ARCT-032 to proceed into a Phase 1b clinical study in CF patients
Investor conference call at
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230807532754/en/
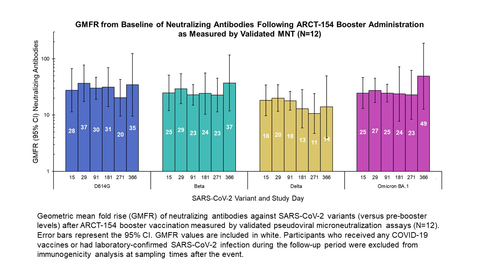
Geometric mean fold rise (GMFR) of neutralizing antibodies against SARS-CoV-2 variants (versus pre-booster levels) after ARCT-154 booster vaccination measured by validated pseudoviral microneutralization assays (N=12). Error bars represent the 95% CI. GMFR values are included in white. Participants who received any COVID-19 vaccines or had laboratory-confirmed SARS-CoV-2 infection during the follow-up period were excluded from immunogenicity analysis at sampling times after the event. (Graphic:
“Arcturus has continued to achieve significant operational progress alongside our exclusive global vaccines partner CSL Seqirus, highlighted by the NDA submission of ARCT-154, for the COVID-19 primary series vaccine and booster, in
Recent Corporate Highlights
-
The primary endpoint was achieved in the ARCT-154 Phase 3 booster vaccine study, demonstrating non-inferiority of immune response against SARS-CoV-2 ancestral strain compared to Comirnaty. Superiority of ARCT-154 in neutralizing antibody response against SARS-CoV-2 Omicron BA.4/5 variant was also demonstrated as a key secondary endpoint.
- The study compared immune responses between ARCT-154 and Comirnaty booster doses (original strain) in Japanese adults (N = 828 and randomized 1:1 to ARCT-154 and Comirnaty) that were previously immunized with two doses of mRNA COVID-19 vaccine then a third booster dose of Comirnaty at least 3 months prior to enrollment.
- ARCT-154, a self-amplifying mRNA vaccine, was administered at 5 mcg, a significantly lower dose relative to Comirnaty (30 mcg).
- Initial study results have been published in MedRxiv. The data suggests increased immunogenicity associated with ARCT-154 at Day 29 with 1.43-fold higher geometric mean titers of neutralizing antibodies against the vaccine strain versus Comirnaty.
- At the time of interim data cut, ARCT-154 was considered safe and tolerable with no safety concerns identified.
-
Meiji Seika Pharma and the Japanese government provided funding for the ARCT-154 Phase 3 booster study. -
Meiji Seika Pharma is responsible for obtaining regulatory approval, distribution, sales and marketing of ARCT-154 inJapan . -
Meiji Seika Pharma announced a collaboration withARCALIS Co., Ltd. to establish integrated cGMP mRNA vaccine manufacturing capabilities, from drug substance to drug product, inJapan .ARCALIS recently announced that construction has been completed for their state-of-the-art manufacturing facility inJapan .
- A Phase 1/2 clinical trial demonstrated one-year durability of immune response following ARCT-154 booster vaccine administration using validated microneutralization (MNT) assays. The geometric mean fold rise (GMFR) in neutralizing antibodies remained greater than 10-fold above baseline for one year across a panel of variants (ref: Figure), including Omicron BA.1.
- The LUNAR-FLU program continues to progress with funding and operational support from CSL Seqirus. LUNAR-FLU utilizes Arcturus’ validated next generation STARR® mRNA platform.
-
In June, the Company announced that the
U.S. Food and Drug Administration (FDA) had granted Fast Track Designation to ARCT-810, the Company’s mRNA therapeutic candidate for ornithine transcarbamylase (OTC) deficiency. The Company has also recently received Rare Pediatric Disease Designation from the FDA for ARCT-810, which is designed to recognize serious or life-threatening manifestations primarily affecting patients under 18 years of age. Due to such designation, if ARCT-810 achieves approval for a pediatric indication in the original rare pediatric disease product application, Arcturus will receive a voucher for priority review of a subsequent marketing application for a different product.-
ARCT-810 Phase 1b single ascending dose study in the
U.S. has completed enrollment and dosing of all cohorts (N = 16 patients). -
ARCT-810 Phase 2 study in
UK andEurope will enroll up to 24 adolescents and adults with OTC deficiency. The ongoing study is evaluating two dose levels and includes up to six (6) bi-weekly administrations for each participant. The Company expects to share interim data on biological activity from a subset of patients in the coming months.
-
ARCT-810 Phase 1b single ascending dose study in the
-
ARCT-032, the Company’s inhaled mRNA therapeutic for cystic fibrosis, has completed dosing in a Phase 1 study in
New Zealand , including 32 subjects across four (4) ascending single-dose cohorts. The Company received regulatory approval of a protocol amendment to allow the transition to a Phase 1b clinical study of ARCT-032 in up to 8 adult cystic fibrosis patients.
Financial Results for the Three and Six Months Ended
Revenues in conjunction with strategic alliances and collaborations:
Arcturus’ primary sources of revenues were from license fees, consulting and related technology transfer fees, reservation fees and collaborative payments received from research and development arrangements with pharmaceutical and biotechnology partners. For the three months ended
Operating expenses:
Total operating expenses for the three months ended
Research and development expenses:
Our research and development expenses consist primarily of external manufacturing costs, in-vivo research studies and clinical trials performed by contract research organizations, clinical and regulatory consultants, personnel related expenses, facility related expenses and laboratory supplies related to conducting research and development activities. Research and development expenses were
General and Administrative Expenses:
General and administrative expenses primarily consist of salaries and related benefits for our executive, administrative, legal and accounting functions and professional service fees for legal and accounting services as well as other general and administrative expenses. General and administrative expenses were
Net Loss:
For the three months ended
Cash Position and Balance Sheet:
Cash, cash equivalents and restricted cash were
Earnings Call:
- Domestic: 1-877-407-0784
- International: 1-201-689-8560
- Conference ID: 13738872
- Webcast: Link
About
Founded in 2013 and based in
Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the likelihood of success of the Company’s pipeline (including ARCT-032 and ARCT-810) and partnered programs (including the COVID-19 and flu programs partnered with CSL Seqirus), the potential of the Company’s platform technology to be meaningfully differentiated from other technologies, the anticipated timing and sharing of clinical data including from the Company’s ARCT-810 and ARCT-032 programs, the continued progress of the LUNAR-FLU program, the likelihood and timing of regulatory approvals of any products including ARCT-154 in
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR®, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
|
CONDENSED CONSOLIDATED BALANCE SHEETS |
||||||||
|
|
|
|
|
|
||||
|
(in thousands, except par value information) |
|
(unaudited) |
|
|
||||
|
Assets |
|
|
|
|
||||
|
Current assets: |
|
|
|
|
||||
|
Cash and cash equivalents |
|
$ |
323,471 |
|
|
$ |
391,883 |
|
|
Restricted cash |
|
|
55,000 |
|
|
|
— |
|
|
Accounts receivable |
|
|
2,799 |
|
|
|
2,764 |
|
|
Prepaid expenses and other current assets |
|
|
3,974 |
|
|
|
8,686 |
|
|
Total current assets |
|
|
385,244 |
|
|
|
403,333 |
|
|
Property and equipment, net |
|
|
12,722 |
|
|
|
12,415 |
|
|
Operating lease right-of-use asset, net |
|
|
30,553 |
|
|
|
32,545 |
|
|
Non-current restricted cash |
|
|
2,127 |
|
|
|
2,094 |
|
|
Total assets |
|
$ |
430,646 |
|
|
$ |
450,387 |
|
|
Liabilities and stockholders’ equity |
|
|
|
|
||||
|
Current liabilities: |
|
|
|
|
||||
|
Accounts payable |
|
$ |
13,619 |
|
|
$ |
7,449 |
|
|
Accrued liabilities |
|
|
28,763 |
|
|
|
30,232 |
|
|
Current portion of long-term debt |
|
|
— |
|
|
|
60,655 |
|
|
Deferred revenue |
|
|
47,963 |
|
|
|
28,648 |
|
|
Total current liabilities |
|
|
90,345 |
|
|
|
126,984 |
|
|
Deferred revenue, net of current portion |
|
|
25,725 |
|
|
|
20,071 |
|
|
Operating lease liability, net of current portion |
|
|
28,111 |
|
|
|
30,216 |
|
|
Other non-current liabilities |
|
|
1,290 |
|
|
|
2,804 |
|
|
Total liabilities |
|
|
145,471 |
|
|
|
180,075 |
|
|
Stockholders’ equity |
|
|
|
|
||||
|
Common stock, |
|
|
27 |
|
|
|
27 |
|
|
Additional paid-in capital |
|
|
625,085 |
|
|
|
608,426 |
|
|
Accumulated deficit |
|
|
(339,937 |
) |
|
|
(338,141 |
) |
|
Total stockholders’ equity |
|
|
285,175 |
|
|
|
270,312 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
430,646 |
|
|
$ |
450,387 |
|
|
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS (unaudited) |
||||||||||||||||
|
|
|
Three Months Ended |
|
Six Months Ended |
||||||||||||
|
|
|
|
|
|
||||||||||||
|
(in thousands, except per share data) |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
||||||||
|
Revenue: |
|
|
|
|
|
|
|
|
||||||||
|
Collaboration revenue |
|
$ |
9,565 |
|
|
$ |
27,093 |
|
|
$ |
89,294 |
|
|
$ |
32,337 |
|
|
Grant revenue |
|
|
954 |
|
|
|
— |
|
|
|
1,510 |
|
|
|
— |
|
|
Total revenue |
|
|
10,519 |
|
|
|
27,093 |
|
|
|
90,804 |
|
|
|
32,337 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
||||||||
|
Research and development, net |
|
|
52,668 |
|
|
|
38,189 |
|
|
|
104,436 |
|
|
|
83,082 |
|
|
General and administrative |
|
|
13,225 |
|
|
|
10,993 |
|
|
|
26,987 |
|
|
|
21,723 |
|
|
Total operating expenses |
|
|
65,893 |
|
|
|
49,182 |
|
|
|
131,423 |
|
|
|
104,805 |
|
|
Loss from operations |
|
|
(55,374 |
) |
|
|
(22,089 |
) |
|
|
(40,619 |
) |
|
|
(72,468 |
) |
|
Loss from equity-method investment |
|
|
— |
|
|
|
(131 |
) |
|
|
— |
|
|
|
(515 |
) |
|
Gain (loss) from foreign currency |
|
|
149 |
|
|
|
1,217 |
|
|
|
(179 |
) |
|
|
1,375 |
|
|
Gain on debt extinguishment |
|
|
— |
|
|
|
— |
|
|
|
33,953 |
|
|
|
— |
|
|
Finance income (expense), net |
|
|
3,252 |
|
|
|
(560 |
) |
|
|
5,729 |
|
|
|
(1,124 |
) |
|
Net loss before income taxes |
|
|
(51,973 |
) |
|
|
(21,563 |
) |
|
|
(1,116 |
) |
|
|
(72,732 |
) |
|
Provision for income taxes |
|
|
577 |
|
|
|
- |
|
|
|
680 |
|
|
|
||
|
Net loss |
|
$ |
(52,550 |
) |
|
$ |
(21,563 |
) |
|
$ |
(1,796 |
) |
|
$ |
(72,732 |
) |
|
Net loss per share, basic and diluted |
|
$ |
(1.98 |
) |
|
$ |
(0.82 |
) |
|
$ |
(0.07 |
) |
|
$ |
(2.75 |
) |
|
Weighted-average shares outstanding, basic and diluted |
|
|
26,563 |
|
|
|
26,425 |
|
|
|
26,557 |
|
|
|
26,401 |
|
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
||||||||
|
Net loss |
|
$ |
(52,550 |
) |
|
$ |
(21,563 |
) |
|
$ |
(1,796 |
) |
|
$ |
(72,732 |
) |
|
Comprehensive loss |
|
$ |
(52,550 |
) |
|
$ |
(21,563 |
) |
|
$ |
(1,796 |
) |
|
$ |
(72,732 |
) |
View source version on businesswire.com: https://www.businesswire.com/news/home/20230807532754/en/
IR and Media Contacts
VP, Head of IR/PR/Marketing
(858) 900-2682
IR@ArcturusRx.com
Kendall Investor Relations
(617) 914-0008
ctanzi@kendallir.com
Source: