Arcturus Therapeutics Announces Second Quarter 2021 Financial Results and mRNA Vaccine and Therapeutics Pipeline Progress
ARCT-021, Arcturus’ single shot STARR™ mRNA COVID vaccine, to begin multinational placebo-controlled Phase 3 efficacy study funded and sponsored by a global entity
ARCT-154, Arcturus’ STARR™ mRNA vaccine candidate targeting COVID variants of concern, elicits robust neutralizing antibody titers against all variants tested in primates, including the Delta variant
ARCT-154 to begin staged Phase 3 study in
Investor conference call at
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210809005702/en/
“This has been an exceptionally productive period where Arcturus has made substantial progress with our mRNA-based vaccine and therapeutic platforms. We are excited about the imminent initiation of the global Phase 3 vaccine trial of ARCT-021, which is funded by a global entity. We made excellent progress advancing and partnering ARCT-154, our next generation vaccine, which targets SARS-CoV-2 variants, including the rapidly expanding and highly transmissible Delta variant. In addition to the progress with our vaccine franchise, we’ve also advanced our therapeutics pipeline, with the approval of a multiple dose Phase 2 study with ARCT-810, our systemically administered mRNA therapeutic candidate for individuals with OTC deficiency,” said
Recent Corporate Highlights
- ARCT-021 has been selected by a global entity for inclusion in a multinational Phase 3 vaccine trial against COVID-19. The placebo-controlled study plans to enroll tens of thousands of participants and will evaluate a 5-mcg dose of ARCT-021 administered as a single injection regimen. The Phase 3 study will be sponsored and funded by the entity.
- Arcturus announced an agreement with Vinbiocare, whereby Vinbiocare will fully fund and establish a facility in
Vietnam for the manufacture of Arcturus’ investigational COVID-19 vaccines. In addition to the$40 million upfront payment, Vinbiocare will purchase the mRNA drug substance from Arcturus and pay a royalty on manufactured doses.
- Arcturus has advanced ARCT-154, our next generation STARR™ mRNA vaccine candidate targeting SARS-CoV-2 variants of concern, toward multiple clinical development studies. Preclinical data demonstrate strong neutralizing immunogenicity in non-human primates to SARS-CoV-2 Alpha, Beta, Gamma, and Delta variants. ARCT-154 elicits 14.4 to 25.9-fold higher neutralizing antibody titers than ARCT-021 in non-human primates, including an observed increase of 17.8-fold higher neutralizing antibody titers against the Delta variant.
ARCT-154 utilizes an optimized STARR™ mRNA with multiple improvements, including modifications for stability and translation, increased immunogenicity of the spike protein antigen via amino acid substitution, expressing the spike protein in a pre-fusion state, and inactivating the furin cleavage site.
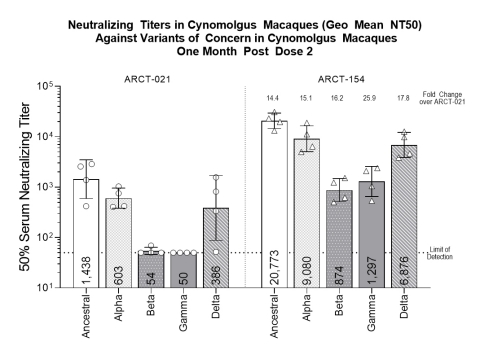
|
Neutralizing Titers (Geo Mean NT50) Against Variants of Concern in Cynomolgus Macaques One Month Post Dose 2 |
|||||
|
STARR™ Vaccine |
Ancestral |
Alpha |
Beta |
Gamma |
Delta |
|
ARCT-021 (7.5 mcg x 2) |
1,438 |
603 |
54 |
50 |
386 |
|
ARCT-154 (7.5 mcg x 2) |
20,773 |
9,080 |
874 |
1,297 |
6,876 |
|
Fold Improvement |
14.4 |
15.1 |
16.2 |
25.9 |
17.8 |
Non-Human Primate (NHP) data collected one month after second dose of 7.5 mcg; analysis of NHP serum was performed using non-replicating vesicular stomatitis virus pseudo-typed with the spike protein of the SARS-CoV-2 variants of concern indicated. Titers (geometric mean) were determined by calculating the dilution that resulted in 50% inhibition of cells expressing GFP encoded by the pseudovirus, a surrogate of virus infection. Error bars indicate geometric standard deviation.
T cell responses for ARCT-021 and ARCT-154 are robust and similar in non-human primates. Notably, STARR™ mRNA vaccines elicit similar T cell responses against all variants of concern tested. The robust T cell responses are attributed to the self-amplifying mRNA mechanism of antigen
expression.
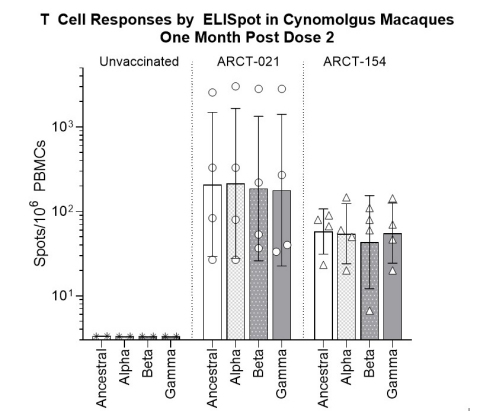
T cell responses from non-human primates assessed one month after second dose of 7.5 mcg; SARS-CoV-2 spike specific T cell responses were analyzed by ELISpot assay using overlapping 15-mer peptides spanning the entire spike antigen from the ancestral SARS-CoV-2 strains or the Alpha, Beta, and Gamma variants of concern. Spot Forming Units (SFU) were determined after background subtraction of unstimulated controls. Bars indicate mean values and error bars indicate standard deviation. (Photo: Business Wire)
- Arcturus announced approval of a Clinical Trial Application (CTA) from the
Singapore Health Sciences Authority (HSA) enabling the advancement of ARCT-154 into a Phase 1/2 clinical trial to evaluate the vaccine as a primary vaccination series and as a booster following initial vaccination with Comirnaty® (marketed by Pfizer and BioNTech). The Phase 1/2 trial costs are funded in part from a previously secured grant fromSingapore .
- Arcturus announced that the company’s partner Vinbiocare received approval for a CTA from the
Vietnam Ministry of Health to advance ARCT-154 into a Phase 1/2/3 clinical study. The trial is a randomized, observer-blind, placebo-controlled design, and is sponsored and completely funded by Vinbiocare. The Phase 1/2/3 study will assess the safety, immunogenicity and efficacy in up to 21,000 adults, with potential Emergency Use Authorization (EUA) by theVietnam Ministry of Health inDecember 2021 .
- Arcturus announced approval from the
UK Health Research Authority to initiate a multiple dose Phase 2 clinical study for ARCT-810, a novel mRNA-based therapeutic candidate for Ornithine Transcarbamylase (OTC) Deficiency. The ARCT-810 Phase 2 study is a randomized, double-blind, placebo-controlled, nested single and multiple ascending dose design for adolescents and adults with OTC deficiency. ARCT-810 Phase 2 study interim results in a subset of participants are expected in H2 2022.
Financial Results for the Quarter Ended
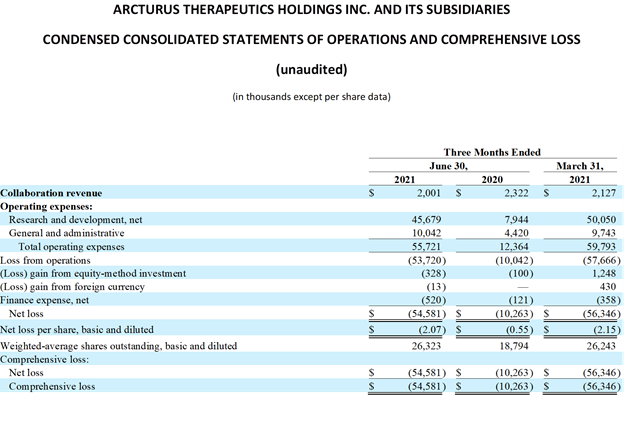
Revenues in conjunction with strategic alliances and collaborations: Arcturus’ primary sources of revenues were from license fees and collaborative payments received from research and development arrangements with pharmaceutical and biotechnology partners. For the three months ended
Operating expenses: Total operating expenses for the three months ended
Research and development expenses increased by approximately
For the three months ended
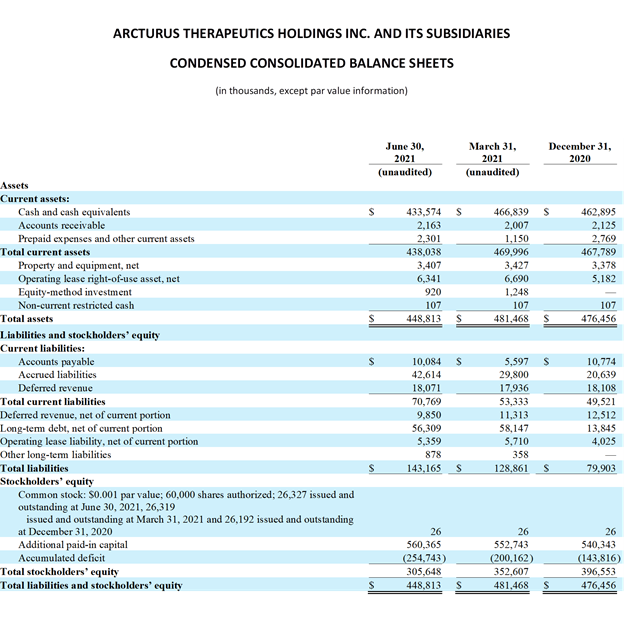
The Company’s cash balance totaled
|
|
|
|
Domestic: |
877-407-0784 |
|
International: |
201-689-8560 |
|
Conference ID: |
13721797 |
|
Webcast: |
|
About
Founded in 2013 and based in
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, collaborations, the planned initiation, design or completion of clinical trials, anticipated sponsorship and/or funding of clinical trials of our candidates, the likelihood that the Company will obtain clearance from regulatory authorities to proceed with planned clinical trials, the ability to enroll subjects in clinical trials, the likelihood that preclinical or clinical data will be predictive of future clinical results, the likelihood that clinical data will be sufficient for regulatory approval or completed in time to submit an application for regulatory approval within a particular timeframe, the anticipated timing for regulatory submissions, the timing of, and expectations for, any results of any preclinical or clinical studies or regulatory approvals, the likelihood of success (including safety and efficacy) of the Company’s pipeline, including ARCT-021, ARCT-154 and ARCT-810, the potential administration regimen or dosage, or ability to administer multiple doses of, any of the Company’s drug candidates, the Company’s efforts to develop a vaccine against COVID-19 and therapeutic potential thereof based on the Company’s mRNA therapeutics, the Company’s manufacturing methods and technologies, the likelihood that a patent will issue from any patent application, its current cash position and adequacy of its capital to support future operations, and the impact of general business and economic conditions. Actual results and performance could differ materially from those projected in any forward-looking statements as a result of many factors including, without limitation, the ability to enroll subjects in clinical trials as a result of the COVID-19 pandemic, the impact of commercialization of third-party COVID-19 vaccines on the design, and ability to conduct, clinical trials, the availability of manufacturing capacity and raw materials, unexpected clinical results, government regulations impacting the regulatory environment or intellectual property landscape, and general market conditions that may prevent such achievements or performance. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties, including those discussed under the heading "Risk Factors" in Arcturus’ Annual Report on Form 10-K for the fiscal year ended
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210809005702/en/
IR and Media Contacts
(858) 900-2682
IR@ArcturusRx.com