Arcturus Therapeutics Announces First Quarter 2022 Financial Update and Pipeline Progress
ARCT-154 booster data show 54- and 46-fold increases in neutralizing antibody response against Omicron BA.1 and BA.2
Investor conference call at
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220509006091/en/
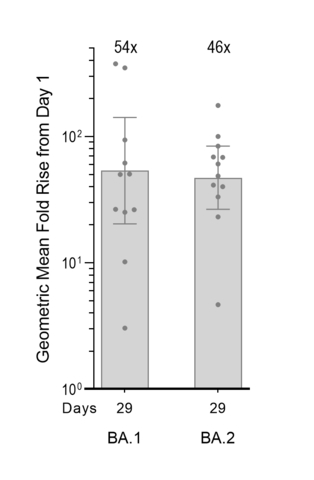
Pseudovirus MNT assay (exploratory assay;
“The first quarter of this year has been commemorated by several key achievements for Arcturus. Our self-amplifying mRNA platform has been the first of its kind to demonstrate meaningful protection against COVID-19 disease in a pivotal primary vaccination trial,” said
Recent Corporate Highlights
- Arcturus today announced neutralizing antibody data from its ongoing Phase 1/2 study evaluating a single 5-mcg booster dose of ARCT-154 given at least five months following two primary doses of Comirnaty®. Pseudovirus microneutralization (MNT) assay on sera samples from twelve participants in the study demonstrate 54-fold and 46-fold geometric mean fold rises (GMFR) in neutralizing antibody titers against the original Omicron BA.1 and currently circulating Omicron BA.2 strains, respectively, on Day 29 post-boost compared to Day 1 pre-boost levels (see Figure).
- Arcturus recently announced three-month durability results from the booster study. Validated pseudovirus MNT assay results demonstrated high levels of neutralizing antibody response against the D614G SARS-CoV-2 strain. Neutralizing antibody responses on Day 15 and Day 29 were 28-fold and 40-fold, respectively, and persisted at 30-fold GMFR three months post-boosting. Validated pseudovirus MNT assay for the Beta strain also demonstrated notable durability of neutralizing antibody response at three months with 24-fold of GMFR. The GMFRs were 26-fold and 31-fold over baseline on Day 15 and Day 29, respectively.
- Surrogate virus neutralization (sVNT) assay results illustrated persistent and broad neutralizing antibody responses against several SARS-CoV-2 variants. Three months following booster dose administration of ARCT-154, sVNT responses remained 13 to 30-fold elevated over baseline pre-boost levels.
-
The Company shared compelling vaccine efficacy data from an ongoing trial evaluating ARCT-154 as a primary vaccine in April. The primary efficacy endpoint was met, and the data also demonstrated remarkable vaccine efficacy of 95.3% (95% CI; 80.4% - 98.9%) in the prevention of severe and fatal COVID-19 disease. The vaccine efficacy was 55.0% (95% CI; 46.9% - 61.9%) for preventing overall COVID-19 disease, which is noteworthy against a backdrop of Delta and Omicron SARS-CoV-2 variant circulation in
Vietnam *. These data from approximately 16,000 participants in the placebo-controlled efficacy study were shared with theVietnam Ministry of Health by Arcturus’ partner and study sponsor Vinbiocare and added to the regulatory data package for EUA application presently under review. -
Primary immunogenicity objective of Phase 1/2/3a portions of the study was met with 98.4% four-fold seroconversion for ancestral (
Wuhan ) strain. - Solicited adverse events in the study have been generally mild or moderate in severity and transient (< 4 days duration) in ARCT-154 vaccinated participants. Unsolicited adverse events in the study have been comparable in the placebo and vaccinated arms. No unexpected safety concerns have been identified. Safety data collection remains ongoing.
-
Technology transfer activities between Arcturus and Vinbiocare continue to mature toward completion of a manufacturing facility in
Hanoi for self-amplifying mRNA vaccines. - ARCT-810, the Company’s mRNA therapeutic candidate for ornithine transcarbamylase (OTC) deficiency will be evaluated in a randomized, double-blind, placebo-controlled, nested single and multiple ascending dose Phase 2 study in 24 adolescents and adults with OTC deficiency. The Company remains on track to initiate dosing of the first participants in the second quarter and obtain interim human proof-of-concept data in the second half of 2022.
- ARCT-032, the Company’s mRNA therapeutic candidate for cystic fibrosis, is on track for a Clinical Trial Application (CTA) filing in Q3 2022.
*References:
https://covariants.org/per-country;
https://covid19.who.int/region/wpro/country/vn;
https://ourworldindata.org (Vietnam Link)
Financial Results for the First Quarter Ended
Revenues in conjunction with strategic alliances and collaborations: Arcturus’ primary sources of revenues were from consulting and related technology transfer fees, license fees and collaborative payments received from research and development arrangements with pharmaceutical and biotechnology partners. Total revenue for the three months ended
Operating expenses: Total operating expenses for the three months ended
Research and development expenses: Research and development expenses for the three months ended
Net Loss: For the three months ended
Cash Position: Cash and cash equivalents were
|
Earnings Call Monday, May 9 @ |
||
|
Domestic: |
888-256-1007 |
|
|
International: |
323-994-2093 |
|
|
Conference ID: |
6122841 |
|
|
Webcast: |
||
About
Founded in 2013 and based in
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the expectations for or likelihood of success of any collaborations, the promise of the company’s platform technologies for multiple types of nucleic acid medicines, the likelihood of success (including safety and efficacy) of the Company’s pipeline (including ARCT-154, ARCT-810, ARCT-032 and a STARR mRNA candidate for influenza), the likelihood that any independent verification by Arcturus, or any regulatory body’s assessment of, of data will be consistent with the information shared by Vinbiocare from the ARCT-154 study in
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
|
CONDENSED CONSOLIDATED BALANCE SHEETS (in thousands, except par value information) |
||||||||
|
|
|
|
|
|
||||
|
|
|
(unaudited) |
|
|
||||
|
Assets |
|
|
|
|
||||
|
Current assets: |
|
|
|
|
||||
|
Cash and cash equivalents |
|
$ |
319,678 |
|
|
$ |
370,492 |
|
|
Accounts receivable |
|
|
3,691 |
|
|
|
3,367 |
|
|
Prepaid expenses and other current assets |
|
|
3,636 |
|
|
|
5,102 |
|
|
Total current assets |
|
|
327,005 |
|
|
|
378,961 |
|
|
Property and equipment, net |
|
|
7,530 |
|
|
|
5,643 |
|
|
Operating lease right-of-use asset, net |
|
|
5,245 |
|
|
|
5,618 |
|
|
Equity-method investment |
|
|
131 |
|
|
|
515 |
|
|
Non-current restricted cash |
|
|
2,077 |
|
|
|
2,077 |
|
|
Total assets |
|
$ |
341,988 |
|
|
$ |
392,814 |
|
|
Liabilities and stockholders’ equity |
|
|
|
|
||||
|
Current liabilities: |
|
|
|
|
||||
|
Accounts payable |
|
$ |
10,014 |
|
|
$ |
10,058 |
|
|
Accrued liabilities |
|
|
19,970 |
|
|
|
23,523 |
|
|
Current portion of long-term debt |
|
|
24,260 |
|
|
|
22,474 |
|
|
Deferred revenue |
|
|
53,062 |
|
|
|
43,482 |
|
|
Total current liabilities |
|
|
107,306 |
|
|
|
99,537 |
|
|
Deferred revenue, net of current portion |
|
|
6,641 |
|
|
|
19,931 |
|
|
Long-term debt, net of current portion |
|
|
39,235 |
|
|
|
40,633 |
|
|
Operating lease liability, net of current portion |
|
|
4,057 |
|
|
|
4,502 |
|
|
Total liabilities |
|
$ |
157,239 |
|
|
$ |
164,603 |
|
|
Stockholders’ equity |
|
|
|
|
||||
|
Common stock: |
|
|
26 |
|
|
|
26 |
|
|
Additional paid-in capital |
|
|
583,382 |
|
|
|
575,675 |
|
|
Accumulated deficit |
|
|
(398,659 |
) |
|
|
(347,490 |
) |
|
Total stockholders’ equity |
|
|
184,749 |
|
|
|
228,211 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
341,988 |
|
|
$ |
392,814 |
|
|
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS (unaudited) (in thousands except per share data) |
||||||||||||
|
|
|
Three Months Ended |
||||||||||
|
|
|
|
|
|
||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2021 |
|
|||
|
Collaboration revenue |
|
$ |
5,244 |
|
|
$ |
2,127 |
|
|
$ |
5,794 |
|
|
Operating expenses: |
|
|
|
|
|
|
||||||
|
Research and development, net |
|
|
44,893 |
|
|
|
50,050 |
|
|
|
32,633 |
|
|
General and administrative |
|
|
10,730 |
|
|
|
9,743 |
|
|
|
10,806 |
|
|
Total operating expenses |
|
|
55,623 |
|
|
|
59,793 |
|
|
|
43,439 |
|
|
Loss from operations |
|
|
(50,379 |
) |
|
|
(57,666 |
) |
|
|
(37,645 |
) |
|
Gain (loss) from equity-method investment |
|
|
(384 |
) |
|
|
1,248 |
|
|
|
(155 |
) |
|
Gain from foreign currency |
|
|
158 |
|
|
|
430 |
|
|
|
(339 |
) |
|
Finance expense, net |
|
|
(564 |
) |
|
|
(358 |
) |
|
|
(525 |
) |
|
Net loss |
|
$ |
(51,169 |
) |
|
$ |
(56,346 |
) |
|
$ |
(38,664 |
) |
|
Net loss per share, basic and diluted |
|
$ |
(1.94 |
) |
|
$ |
(2.15 |
) |
|
$ |
(1.47 |
) |
|
Weighted-average shares outstanding, basic and diluted |
|
|
26,376 |
|
|
|
26,243 |
|
|
|
26,359 |
|
|
Comprehensive loss: |
|
|
|
|
|
|
||||||
|
Net loss |
|
$ |
(51,169 |
) |
|
$ |
(56,346 |
) |
|
$ |
(38,664 |
) |
|
Comprehensive loss |
|
$ |
(51,169 |
) |
|
$ |
(56,346 |
) |
|
$ |
(38,664 |
) |
View source version on businesswire.com: https://www.businesswire.com/news/home/20220509006091/en/
IR and Media Contacts
(858) 900-2682
IR@ArcturusRx.com
Kendall Investor Relations
(617) 914-0008
ctanzi@kendallir.com
Source: