Arcturus Reports Strong Three-Month Durability Results from ARCT-154 Booster Trial
Three-month data from ARCT-154 booster trial show persistent neutralizing antibody activity against multiple variants of SARS-CoV-2
International pivotal Phase 3 booster trial preparations initiated with global CRO
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220505005501/en/
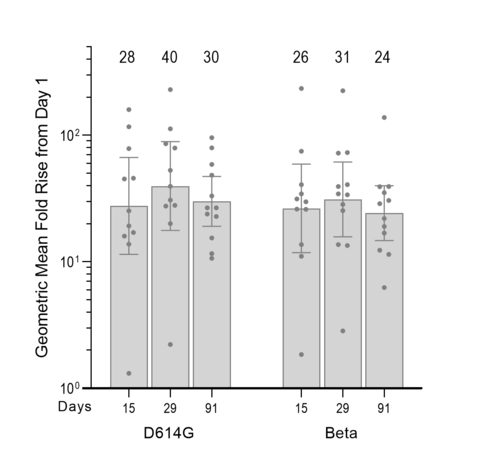
Figure 1: Validated pseudovirus microneutralization (MNT) assay results (left: D614G; right: Beta), showing GMFR levels of neutralizing antibody responses over Day 1 (baseline levels prior to boosting with ARCT-154) calculated with virus neutralization concentrations (with 95% confidence intervals) obtained for participants (for D614G: n = 12/12 for Days 1, 91 and 11/12 for Days 15 29; For Beta: n = 12/12 for Days 1, 29, 91 and 11/12 for Day 15). (Graphic: Business Wire)
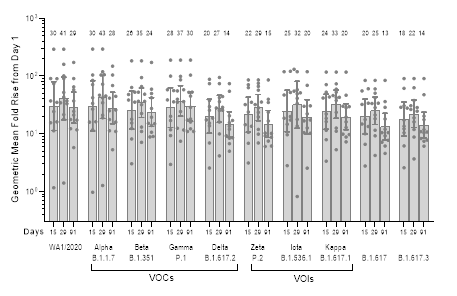
Figure 2: Surrogate virus neutralization (sVNT) assay results for SARS-CoV-2 variants. The panel shows GMFR on Days 15, 29, and 91 over Day 1 (pre-boost baseline levels; n = 12/12 for Days 1, 29, 91; n = 11/12 for Day 15). VOCs = Variants of Concern; VOIs = Variants of Interest
“We are very pleased to see our self-amplifying mRNA vaccine ARCT-154 demonstrate meaningful and durable increases of neutralizing antibodies against SARS-CoV-2 and variants of concern – for at least three months,” said
In the ARCT-154 arm of the ongoing Phase 1/2 booster study in the
Similar to the microneutralization responses, surrogate virus neutralization (sVNT) assay results evaluating a panel of SARS-CoV-2 strains (Figure 2) also illustrated persistent and broad neutralizing antibody responses. Three months following booster dose administration of ARCT-154, antibody responses remained 13 to 30-fold elevated over Day 1 (pre-boost) baseline for all SARS-CoV-2 strains tested. Samples from the trial will be further analyzed for neutralizing antibody activity against different strains of SARS-CoV-2, including the Omicron lineage.
The Company has initiated start-up activities with an international CRO toward a pivotal Phase 3 booster trial intended to support global registration.
About
Founded in 2013 and based in
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the expectations for or likelihood of success of any collaborations, the promise of the company’s platform technologies for multiple types of nucleic acid medicines, the likelihood that ARCT-154 will be advanced or receive any regulatory approval, the likelihood that the ARCT-154 data from the booster study, or any other non-clinical or clinical data, will be predictive of future clinical results, efficacy, durability for longer periods of time or results against COVID variants, the likelihood that ARCT-154 data will be sufficient for any regulatory approval, the timing, design (including number of participants), initiation, conduct or completion of a pivotal booster study of ARCT-154 or any other clinical trial, the Company’s expectation of sharing further analysis from evaluation of sera samples, the likelihood that a patent will issue from any patent application, and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading "Risk Factors" in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220505005501/en/
IR and Media Contacts
Senior Director, Investor Relations
(858) 900-2682
IR@ArcturusRx.com
Kendall Investor Relations
(617) 914-0008
ctanzi@kendallir.com
Source: